Standard Terminology and Format for Labeling of Volumetric Measures on OTC Pediatric Orally Ingested Liquid Drug Products
1. Summary
In 2008, the Centers for Disease Control and Prevention (CDC) convened a stakeholder meeting to share information and expertise on medication overdoses in children. One of the key initiatives defined by the PROTECT group was to refine dosing measures on product labeling to reduce the possibility of unintentional medication overdose. Use of nonstandard dosing devices (e.g., kitchen spoons) or inconsistent dosing directions on product labeling can result in consumer confusion and administration of an inappropriate medication dose.
As a direct result of the PROTECT initiative, CHPA developed a voluntary guideline for industry suggesting ways to standardize volumetric measures in dosing directions and dosing devices for oral pediatric liquid drug products, including preferred use of “mL” as the unit of measure for dosing instructions. Other recommendations provided in the 2009 CHPA guideline were consistent with those in a concurrently released FDA guidance on OTC dosage delivery devices (finalized in 2011).
CHPA is updating voluntary labeling guidelines for liquid products intended to be given to children under 12 years (previously approved in November 2009). Key changes include deletion of spoon labeling (i.e., teaspoon, tablespoon) in dosing directions and on dosing devices; specifying use of “mL” only in dosing directions and on devices; and deletion of the volumetric unit of measure definition (i.e., mL = milliliter). These changes are based on recent activity from FDA (issued a Draft Guidance on pediatric liquid acetaminophen products specifying that dosing directions be provided in mL only), the National Council on Prescription Drug Programs (issued a White Paper recommending that mL be the standard unit of measure for liquid prescription products), and the CDC (which through the PROTECT initiative encourages the adoption of an mL only standard for dosing directions and devices).
2. Objective and Scope
To improve patient safety by decreasing the potential for overdoses, underdoses and other errors when patients or caregivers measure and administer orally ingested OTC liquid medications, these guidelines identify and support consistent terminology, format, and text for volumetric measures within the dosing directions on the outer packaging, the immediate container label, and the dosing device for OTC orally ingested liquid drug products intended for use in children, defined as <12 years of age. Products covered by this voluntary guidance include those marketed pursuant to an OTC Monograph as well as those approved via a New Drug Application (NDA) or Abbreviated NDA (ANDA). Implementation of these guidelines, once approved as part of a members’ label and packaging change process, may take up to several years.
Although similar principles may apply, this document does not address other OTC liquid products such as oral medications indicated for adults and children 12 years and over, prescription medicines or dietary supplements. In addition, the guidance does not address products with children’s dosing intended for topical or non-ingested use such as crèmes or pastes, gargles/mouth rinses or sprays.
3. Background
Communications exist for parents and caregivers about the best ways to give medicines to children, especially the proper use of oral liquid medicines (2-7). Key points provided to parents and caregivers are to always read the label carefully, use the dosing device that comes with the product and to understand the types of liquid measure units for dosing liquid medicines. The use of preferred volumetric measure terms, units and abbreviations, as well as potential areas to avoid has also been suggested (7-14).
In response to reports of unintentional overdoses attributed at least in part to products with confusing or inconsistent labels and measuring devices, FDA released a draft voluntary guideline addressing dosage delivery devices for OTC liquid drug products in November 2009. The FDA voluntary guidance for industry (Dosage Delivery Devices for Orally Ingested OTC Liquid Drug Products) which was finalized in May 2011 provided specific recommendations for aligning dosing devices with the accompanying dosing directions for orally ingested OTC liquid medications (15). In October 2014, FDA also released a draft guidance addressing medication errors and unintentional ingestions of pediatric drug products containing acetaminophen (16). Other authoritative bodies have also released guidance on best practices for reducing medications errors, including those associated with orally ingested liquids (17-21).
In 2009, CHPA conducted an industry-wide survey of OTC oral liquid drug products with dosing directions for children in order to determine potential areas for improving the consistency and standard formatting of volumetric measures. A number of improvements were suggested including standardization of abbreviations, decimals and fractions; representation of volumetric measures in a dosing chart; use of a dosing device (provided with the product); and consistency in volumetric measures between the dosing device and the labeling dosing directions. These recommendations were provided in the CHPA voluntary guideline released in November 2009.
At the time the FDA guidelines were released, a published analysis of product labeling for marketed pediatric oral liquid OTC medications with dosing information for children younger than 12 years found numerous instances of variable dosing directions and inconsistency between dosing directions and measuring devices (22). A more recent study assessed adherence to recommendations provided in the FDA and CHPA guidelines aimed at reducing dosing errors among national brand name orally ingested OTC liquid pediatric medications (23). Recommendations included those which directly addressed potential dosing errors of ≥3-fold (e.g., do not use atypical units, include a dosing device, do not use trailing zeroes, etc.).
Results from this study demonstrated a high level of adherence to the recommendations. Additional opportunities for standardization were noted by the authors including promotion of milliliter (mL) as the standard unit for dosing orally ingested liquid medications as well as the design and marking of dosing devices.
A recently released white paper from the National Council for Prescription Drug Programs (NCPDP — Recommendations and Guidance for Standardizing the Dosing Designations on Prescription Container Labels of Oral Liquid Medications, March 2014) provided recommendations and guidance for standardizing the dosing designation used on prescription container labels of oral liquid medications (24). Recommendations included use of milliliter (mL) as the standard unit of measure, a practice shown to reduce dosing errors (25); use of leading zeros before the decimal point for dosage amounts less than one and avoidance of the use of trailing zeros after a decimal point; and use of dosing devices with numeric graduations and units that correspond to the container labeling.
4. Specific Recommendations
The following recommendations address the labeling dosing directions on the outer packaging, the immediate container labeling, and the dosing device, for OTC orally ingested liquid drug products with dosing directions for children.
4.1 OTC Drug Facts Dosing Directions: Outer Package and Immediate Container Labeling
A. Dosing Directions:
Provide a statement(s) that:
- encourages a consumer to select the right dose
- use the dosing device that accompanies the product
- keep dosing device with product/do not discard dosing device
Example dosing directions (see also Appendix)
“Find right dose on chart. Use only enclosed [insert specific name of product’s dosing device (e.g., “dosing cup”, “oral syringe”)] specifically designed for use with this product. Do not use any other dosing device.).”
B. Dosing Directions: Guidelines for Volumetric Measures
- Use a tabular format to provide dosing directions (if space permits)
- Use milliliter (mL) as the only unit of measure in the dosing directions (e.g., 5 milliliter or 5 mL)
- Avoid use within labeling dosing directions of the following: teaspoon, tablespoon, cubic centimeters, cc, dram, fluid ounce, Fl. Oz., and dropper(ful) or any other less common or nonstandard volumetric measures.
- For fractional volumes, use a decimal; if <1 mL volume, use decimal with a leading zero (e.g., 0.5 mL) to help avoid 10-fold dosing errors. Avoid use of trailing zeros after a decimal (i.e., use 1 mL not 1.0 mL) to help avoid 10-fold dosing errors.
4.2 Dosing Device: Dosing Device Accompanying the Product
A. Dosing Device: Guidelines for Volumetric Measures
- Provide a calibrated dosing device with all orally-ingested liquid products.
- Dosage delivery devices should not be significantly larger than the largest dose described in the labeled dosage directions and should permit clear measurement and delivery of the smallest labeled dosage.
- Provide graduated markings on the dosing device that include dosage(s) specified in the dosing directions.
- Use contrasting graduated markings (e.g., etched or printed) so as to aid the readability of the measured liquid.
- Use the milliliter (mL) volumetric unit(s) of measure only.
- For fractional volumes, use the same decimal format and style provided in the dosing directions.
5. Appendix: Examples
5.1 Examples: Dosing Directions Statement(s)
Example A
“Measure the dose correctly using the enclosed [insert specific name of product’s dosing device, e.g., dosing cup, oral syringe]”
Example B
“For accurate dosing, use the enclosed [insert specific name of product’s dosing device, e.g. dosing cup, oral syringe] to measure a dose”
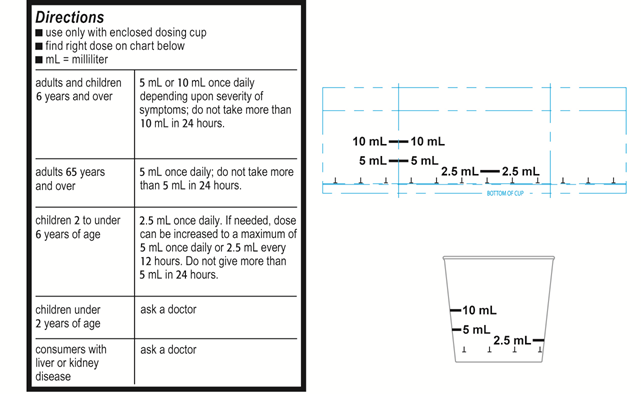
Example C Label statement using only mL (infant acetaminophen products)
“Find right dose on chart below”
“Use only enclosed [insert specific name of product’s dosing device, e.g., dosing cup, oral syringe] designed for use with this product. Do not use any other dosing device.”
5.2 Examples: OTC Drug Facts Directions
Example A
Drug Facts
Directions
| |
| adults and children 6 years and over | 10mL once daily; do not take more than 10 mL in 24 hours. |
| adults 65 years and over | 5 mL once daily; do not take more than 5 mL in 24 hours |
| children 2 to under 6 years of age | 2.5 mL once daily; do not give more than 2.5 mL in 24 hours |
| children under 2 years of age | do not use |
Example B
Drug Facts
Directions
| |
| adults 65 years and over | 15 mL once daily; do not take more than 15 mL in 24 hours. |
| children 2 to under 6 years of age | 7.5 mL once daily; do not give more than 7.5 mL in 24 hours. |
| children under 2 years of age | do not use |
Example C
Drug Facts
Directions
| ||
| Weight (lb) | Age (yr) | Dose (mL) |
| Under 24 | Under 2 | Call a doctor |
| 24-35 | 2-3 | 5 mL |
Attention: specifically designed for use with enclosed dosing device. Do not use any other dosing device with this product.
6. References
- Johnson KB, Lehmann CU; Council on Clinical Information Technology of the American Academy of Pediatrics. Electronic prescribing in pediatrics: toward safer and more effective medication management. Pediatrics. 2013;131(4)
- United States Food and Drug Administration. Medicines in My Home. https://www.fda.gov/drugs/understanding-over-counter-medicines/medicines-my-home-mimh
- American Academy of Pediatrics. How to Use Liquid Medications http://www.healthychildren.org/English/safety-prevention/at-home/medication-safety/Pages/Using-Liquid-Medicines.aspx
- FamilyDoctor.Org. OTC Medicines: Know Your Risks and Reduce Them. https://familydoctor.org/otc-medicines-know-your-risks-and-reduce-them/
- United States Food and Drug Administration. Giving Medication to Children: Q&A with Dianne Murphy, M.D. http://www.fda.gov/downloads/ForConsumers/ConsumerUpdates/UCM164439.pdf
- Consumer Healthcare Products Association. Treat with care – Kids and OTC cough and cold medicines.http://otcsafety.org/uploads/files/publications/Treat_With_Care.pdf
- Shah R, Blustein L, Kuffner E, Davis L. Communicating doses of pediatric liquid medicines to parents/caregivers: a comparison of written dosing directions on prescriptions with labels applied by dispensed pharmacy. J Pediatr. 2014;164(3):596–601
- United States Pharmacopeia. USP Quality Review. July 2004; No.80. http://www.usp.org/usp-healthcare-professionals/medication-safety-labeling
- Institute for Safe Medication Practices. List of Error-Prone Abbreviations, Symbols, and Dose Designations. 2007. http://www.ismp.org/Tools/errorproneabbreviations.pdf
- USP-NF Online – 8.240. Weights and Measures and 1221 General Information
- FDA Consumer Magazine. Avoiding Problems: Liquid Medicines and Dosing Devices. October 1994
- Litovitz T. Implication of Dispensing Cups in Dosing Errors and Pediatric Poisonings: a report from AAPCC. Annals of Pharmacotherapy 1992:26;917-18.
- FDA Guidance for Industry Labeling OTC Human Drug Products — Questions and Answers. December 2008 http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078792.pdf
- Federal Register 21 CFR 201. FDA Over-The-Counter Human Drugs; Labeling Requirements. Final Rule. March 17, 1999 64:51. Example 2 and 3. Pg 13298 and 13299. https://www.govinfo.gov/content/pkg/FR-1999-03-17/pdf/99-6296.pdf
- FDA Guidance for Industry - Dosage Delivery Devices for Orally Ingested OTC Liquid Drug Products, May 2011
- FDA Guidance for Industry – Over-the-Counter Pediatric Liquid Drug Products Containing Acetaminophen, October 2014
- Institute for Safe Medication Practices. ISMP statement on use of metric measurements to prevent errors with oral liquids. Horsham, PA: Institute for Safe Medication Practices; October 2011. www.ismp.org/pressroom/PR20110808.pdf
- United States Pharmacopeia. General notices and requirements applying to standards, test, assays, and other specifications of the United States Pharmacopeia: USP 34
- American Society of Health-System Pharmacists (ASHP). ASHP guidelines on preventing medication errors in hospitals. Am J Hosp Pharm. 1993;50(2):305–314
- National Coordinating Council for Medication Error Reporting and Prevention. Recommendations to enhance accuracy of prescription writing.nccmerp.org
- American Academy of Family Physicians. Preferred unit of measurement for liquid medications. www.aafp.org/about/policies/all/preferred-unit.html
- Yin HS, Wolf MS, Dreyer BP, Sanders LM, Parker RM; Evaluation of Consistency in Dosing Directions and Measuring Devices for Pediatric Nonprescription Liquid Medications, JAMA 2010, 304(23):2595-2602.
- Budnitz DS, Lovegrove MC, Rose KO; Adherence to Label and Device Recommendations for Over-the-Counter Pediatric Liquid Medications, Pediatrics 2014, 133(2): e283-90.
- National Council for Prescription Drug Programs NCPDP Recommendations and Guidance for Standardizing the Dosing Designations on Prescription Container Labels of Oral Liquid Medications Version 1.0; March 2014 https://www.ncpdp.org/NCPDP/media/pdf/wp/DosingDesignations-OralLiquid-MedicationLabels.pdf
- Yin HS, Dreyer BP, Ugboaja DC, Sanchez DC, Paul IM, Moreira HA, Rodriguez L, Mendelsohn AL; Unit of measurement used and parent medication dosing errors, Pediatrics 2014, 134(2) e354-61.